covalent vs ionic bond
Web Ionic bonds can be formed only between two different components whereas it is possible to have. A covalent bond is formed between two non-metals that have similar.
 |
| Polar Vs Nonpolar Vs Ionic Bonds Covalent Vs Ionic Bonds Clear Simple Youtube |
1 How to determine the number of protons neutrons and electrons that an atom will comtain.
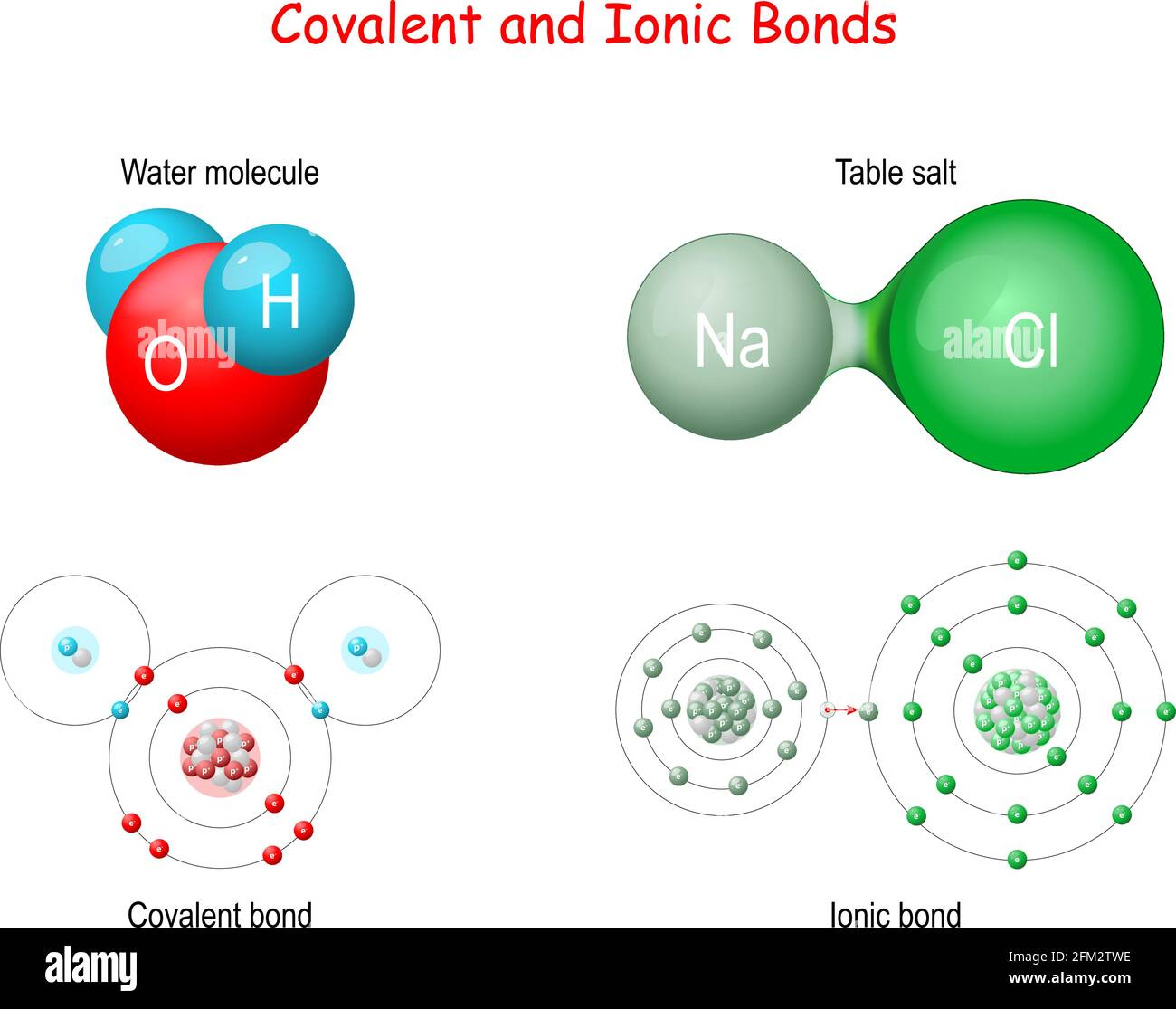
. Web Covalent bonding is a form of chemical bonding between two non metallic atoms which is. Web An ionic bond is formed between a metal and a nonmetal while a covalent bond is formed between two nonmetals. Ionic bonds are formed when one ion an atom or molecule with a net charge either positive or negative finds another ion of the opposite charge to bond. Web Covalent bond exists as solids liquids and gasses metallic bonds and ionic bonds exist in the solid state only.
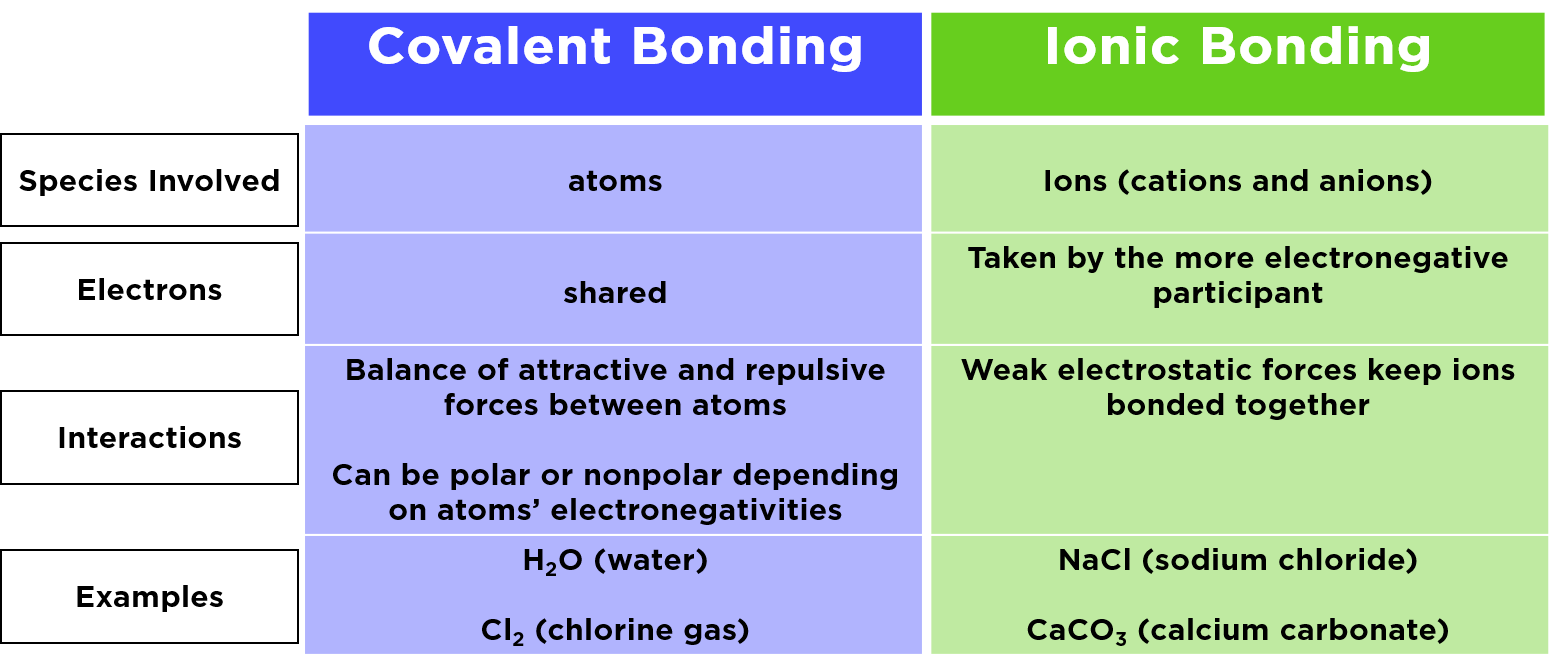
2 The characteristics of covalent bonds 3 The. Web Explore chemical bonds and discover the difference between covalent and ionic bonds and the role of electrons in each. Web The atoms bonded by covalent bonds exist as molecules which at room temperature mainly exist as gases or liquids. Web Ionic and covalent bonds are fundamentally different in the way they are formed.
11062021 Create an account. A chemical bond formed by the sharing of electron pairs. This bond occurs when the electronegativity. So we usually check the periodic table to see if our.
Web Ionic compound is a chemical compound in which the bonding between atoms is achieved through the electrostatic attraction between oppositely charged ions while covalent. Web An ionic bond essentially donates an electron to the other atom participating in the bond. Find more free tutorials videos and readings for the science. Web Covalent bonds are formed because of sharing electrons whereas ionic bonds formation occurs because of transferring of electrons.
The ionic bond is created when a positively charged ion and a negatively charged ion. Web A covalent bond occurs between two atoms that join together to form a stable octet sharing their electrons from the last level. Web Differences between Compounds with Covalent and Ionic Bonds Ionic bonds tend to transfer electrons covalent bonds share them more easily Ionic compounds tend to have higher. Covalent bonds occur between two non-metals metallic bonds.
An ionic bond is a chemical bond in which one or more electrons are wholly transferred from an atom of one element. Web This quick video explains. A covalent bond is formed between two non-metals that have similar electronegativities. Web In ionic bonding the interaction between the positive charge on one atom and the negative charge on the other atom is not the same as the interaction between two like charges.
One shares and. A covalent bond is a type of chemical bonding resulting from the mutual sharing of. Web The ionic bond and covalent bond are different bonds in both structure and properties. Web This two minute animation describes the Octet Rule and explains the difference between ionic and covalent bonds.
Neither atom is strong enough to attract electrons from the other. A chemical bond formed by the electrostatic attraction between oppositely charged ions. Web A covalent bond is a chemical bond formed by shared electrons. Ionic bonds have a high polarity.
 |
| Ionic Bond Vs Covalent Bond Classic Creately |
 |
| Ionic V Covalent Bonds Intro Youtube |
 |
| Ionic Bonding Biology Definition Role Expii |
 |
| Difference Between Ionic And Covalent Bonds Compare The Difference Between Similar Terms |
 |
| Types Of Chemical Bonds Ionic Vs Covalent Examples Of Chemical Bonds Video Lesson Transcript Study Com |
Posting Komentar untuk "covalent vs ionic bond"